

The relative mass of protons is 1, which is approximately equal to the mass of hydrogen (1.00757 amu). When a hydrogen atom removes an electron from its orbit, the positively charged particle that remains is called proton. It resides in the center or nucleus of the atom. Protons are the permanent core particles of an atom. Therefore, the number of negatively charged electrons orbiting in its orbit is equal to the number of positively charged protons in the nucleus.Ītomic number (Z) = Number of charges in the nucleus (p) How many protons does a chlorine atom have? That is, the atomic number is the total number of protons. We know that protons are located in the nucleus of an atom as a positive charge.
ELEMENT CL PROTONS SERIAL NUMBER
This number is equal to the serial number of the periodic table. The atomic number of the element is expressed by ‘Z’. Thus, the number of positive charges present in the nucleus of an element is called the atomic number of that element. He called that number the order of the atoms. The results of his experiments show that each element has a unique integer equal to the number of positive charges in the nucleus of that element. Scientist Henry Gwynn Jefferies Mosle examined the X-ray spectrum of various elements in 1913-to 1914.
ELEMENT CL PROTONS HOW TO
How to easily find the number of electrons, protons and neutrons in a chlorine atom? Electrons revolve around the nucleus in a specific orbit. The only exception is hydrogen, which has only protons in its nucleus but no neutrons.
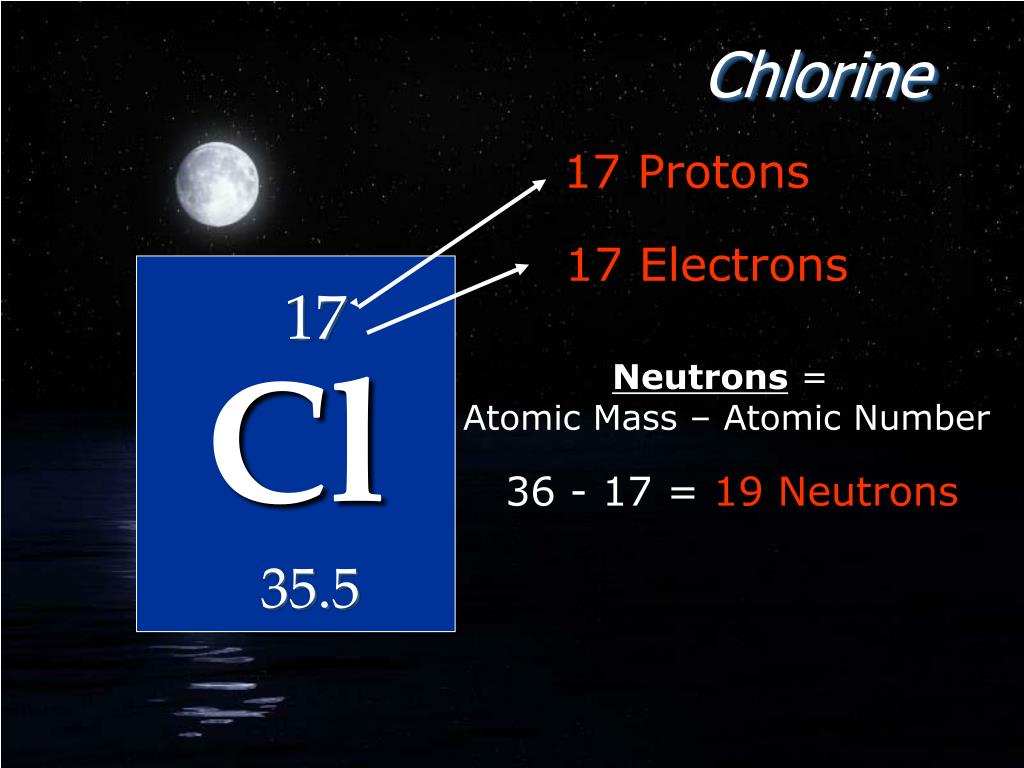
Experiments by various scientists have shown that the nucleus of an atom contains protons and neutrons. One is the nucleus and the other is the orbit. Also, neutrino, antineutrino, positron, and mason are located in an atom as temporary particles.Ītoms can usually be divided into two parts. Numerous permanent and temporary particles exist in the atom.Įlectrons, protons, and neutrons are located in the atom as permanent particles. However, it has been possible to detect atoms by increasing the vision of a very powerful electron microscope by two million times. So, if 1000 crore atoms of hydrogen are arranged side by side, it will be 1 meter long. The diameter of an atom of hydrogen is 0.1nm (1.0nm = 10 -9m). Atoms are so small particles that they cannot be seen even under a powerful microscope. Where are the electrons, protons and neutrons located in an atom?Īn atom is the smallest particle of an element that has no independent existence but is directly involved in chemical reactions as the smallest unit. Hopefully, after reading this article you will know the details about this topic. This article discussed in detail how to easily find the number of protons, neutrons, and electrons in a chlorine atom.Īlso discussed is the position of electrons, protons, and neutrons in an atom, the number of atomic masses, and the isotopes of chlorine. The chlorine atom has two stable isotopes. The number of neutrons depends on the isotope of the element. Therefore, a chlorine atom has eighteen neutrons. The difference between the mass number of the chlorine atom and the number of protons is eighteen. The number of neutrons in an atom can be determined by the difference between the atomic mass and the number of protons. Therefore, a chlorine atom has seventeen protons and seventeen electrons. The atomic number of an element is equal to the number of protons and electrons in that element. Chlorine is the 17th element of the periodic table so its atomic number is 17. The price of pure chlorine may vary between $0.15 and $0.16.Chlorine is a classified halogen element and its symbol is Cl. Salt water can be electrolyzed to release chlorine. It was used as a chemical weapon in the World War I by the Germans for its toxic nature. Inhalation of high concentration of the gas can lead to severe health hazards inhalation like pulmonary edema.
ELEMENT CL PROTONS SKIN
Prolonged exposure to chlorine gas can cause watery eyes, skin rash, burns, shortness of breath, nausea, and vomiting. It finds application in certain oxidation and substitution reactions.Some medicines and pharmaceutical products used in the treatment of arthritis, allergy, and high cholesterol contain chlorine as the active ingredient.The element is used in the manufacture of several consumer products like paints, dyes, solvents, paper, insecticides, and textiles.Commercial household bleaches containing Cl are effective in cleaning kitchen and bathroom areas, and whitening clothes.It is added to PVC (polyvinyl chloride) used in electrical wiring insulation, window frames, water pipes, blood bags, and car interiors.Chlorine-based liquids, granules, and tablets are used as disinfectants to treat swimming pools and drinking water.
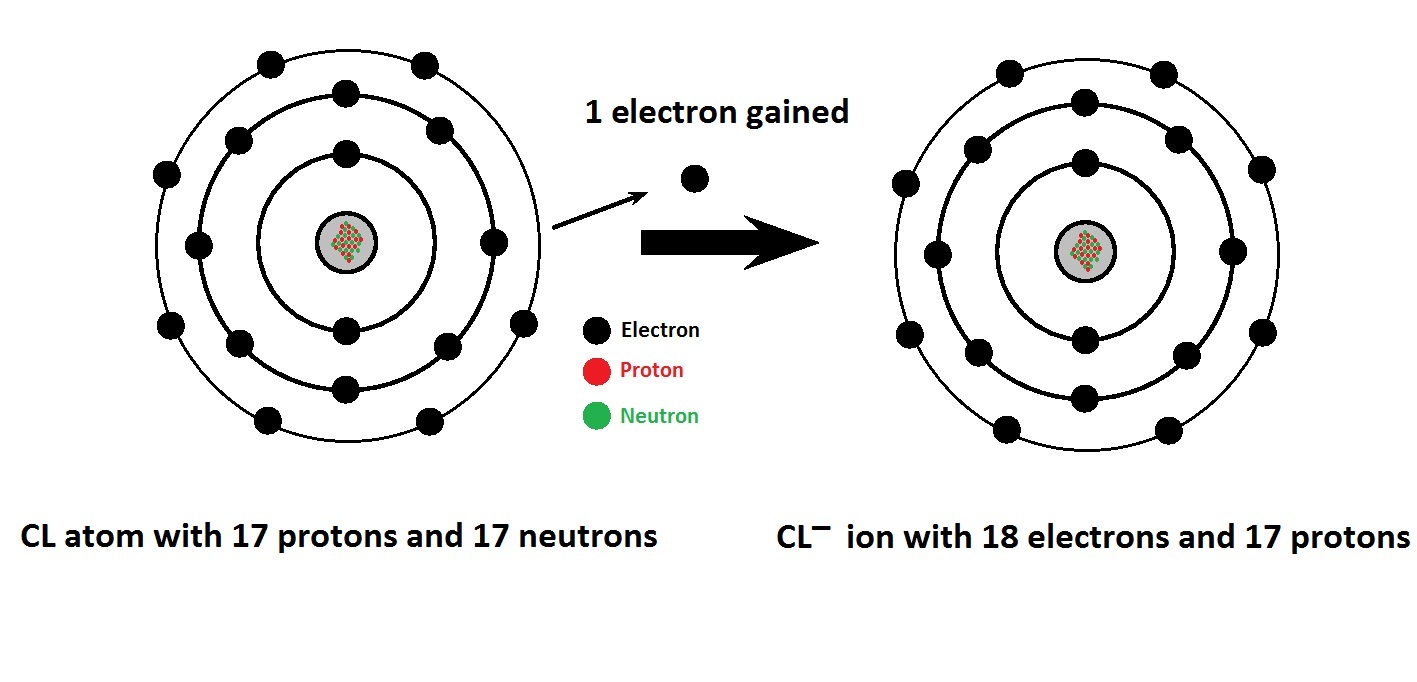
Chlorine Atomic Structure (Bohr Model) What are the Common Uses of Chlorine Gas


 0 kommentar(er)
0 kommentar(er)
